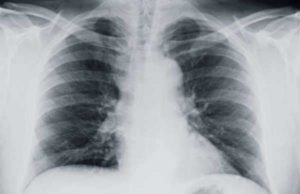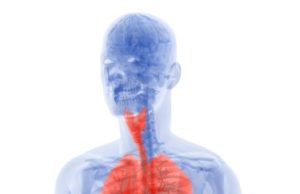
Mesothelioma is a rare form of cancer that affects the mesothelium, which a lining that serves as protection to the human body’s internal organs. Most commonly, mesothelioma will affect the lungs, notably the pleural lining. However, mesothelioma can also develop in the lining of the abdominal cavity, the peritoneum, the lining of the heart, the pericardium, and in the tunica vaginalis.
What Causes Mesothelioma?
Mesothelioma is almost exclusively related and caused to the exposure of asbestos, a material that is used in a variety of application, particularly insulation, cement mixing, and in the automotive brakes.
The risk associated with developing mesothelioma arises from the prolonged inhalation of asbestos particles or dust. Due to the different uses or applications of asbestos, many people are at risk of developing mesothelioma, particularly if their profession entails working with, handling, or being in the presence of asbestos.
However, the dangers associated with asbestos exposure were not known until the mid to late 1970s, well after the material was used in various and different industries. For many that are diagnosed with disease, the symptoms of mesothelioma do not appear well after the initial exposure to asbestos, often times well after working in professions that involved the presence of asbestos.
What are the Types of Mesothelioma?
There are four known types of mesothelioma that exist, each one named after the particular area or organ they affect. The types of mesothelioma are:
1. Pleural Mesothelioma: Affects the lining of the lungs, also referred to as the pleura or pleural lining. This is the most common type of mesothelioma, affecting about 70% to 75% of patients diagnosed with the disease.
2. Peritoneal Mesothelioma: Develops in the peritoneal lining, which is the protective lining of the abdominal cavity. This type of mesothelioma is the second most commonly diagnosed, present in about 20% of all mesothelioma patients.
3. Pericardial Mesothelioma: Formed in the protective sac that surrounds the heart, which is the pericardium. This type of mesothelioma is rare when compared to the incident rates, occurring in only about 5% of all mesothelioma cases.
4. Testicular Mesothelioma: Develops in the tunica vaginalis of the testicles and is the rarest type of mesothelioma, with only about 100 cases reported in total in the past 60 years.
Legal Issues Regarding Mesothelioma
After the dangers involving the exposure to asbestos being related as the main cause of mesothelioma, there were many disputes in the courts regarding compensation and damages.
The fact is that even though reports regarding the hazards of asbestos to human health were made public in the 1970s, knowledge of the possible risks was well known prior. Many companies that used asbestos products often times failed to properly warn their employees of the inherent dangers and failed to take the necessary precautions and safety procedures to safeguard their workers.
Because of such inaction, many companies were held liable for their negligence to properly provide for the health and well-being of their workers. Even today, mesothelioma and asbestos remains a common issue or subject of litigation in the United States.
In the case that a person is diagnosed with mesothelioma, it is important to seek legal advice to determine if any compensation is due as the result of negligence on behalf of an employer or company.